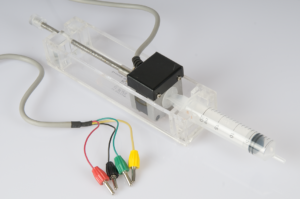
Driving question : How can you determine a concentration of an unknown acidic solution?
In this activity students carry out a titration to determine the concentration of an acidic solution. The equivalence point can be determined from the titration curve. Compared to a “regular” titration, the use of an automated step motor burette adds an extra dimension to the experiment, allowing for addition of the base using constant speed. This experiment focusses on setting up the experiment and analysing the results.
Topic: Acids and bases,
Concepts: Acid, Acid-base reaction, Base, Chemical calculations, Equivalence point, Neutralization of acid, pH, Titration,
Grade level: High School (14-18 years),
Activity type: Data logging,
Sensors: pH sensor, Step motor burette,
Interface requirements: Interface with actuator output,
The Student Worksheet gives investigation instructions for students.
The Teacher Notes includes teacher commentaries such as didactical approach, exemplary questions, assignments and data analysis.
The Science Background describes the scientific principles that underlie the activity.
Coach Activity/Result file can be opened in the Coach 7 Program. This requires Coach 7 to be installed on your computer.
Download activity
Titration with step motor burette (.cma7)
Download result
Titration with step motor burette (.cmr7)
Download activity
Volume calibration for step motor burette (.cma7)