High School (14-18 years)
 In this activity students study an existing model of an irreversible reaction of N2O4 to NO2 and expand it in order to create a chemical equilibrium. In order to teach students to critically assess a model, the concentration quotient has to be calculated at some point in the activity. This activity focusses on the essence of modelling and less on the workings of an equilibrium.
Activity Gasses in equilibrium
In this activity students study an existing model of an irreversible reaction of N2O4 to NO2 and expand it in order to create a chemical equilibrium. In order to teach students to critically assess a model, the concentration quotient has to be calculated at some point in the activity. This activity focusses on the essence of modelling and less on the workings of an equilibrium.
Activity Gasses in equilibrium
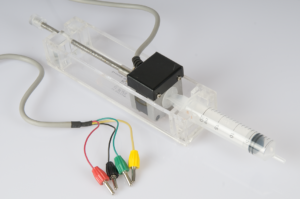 In this activity students carry out a titration to determine the concentration of an acidic solution. The equivalence point can be determined from the titration curve. Compared to a “regular” titration, the use of an automated step motor burette adds an extra dimension to the experiment, allowing for addition of the base using constant speed. This experiment focusses on setting up the experiment and analysing the results.
Activity Titration with step motor burette
In this activity students carry out a titration to determine the concentration of an acidic solution. The equivalence point can be determined from the titration curve. Compared to a “regular” titration, the use of an automated step motor burette adds an extra dimension to the experiment, allowing for addition of the base using constant speed. This experiment focusses on setting up the experiment and analysing the results.
Activity Titration with step motor burette
 In this activity students determine the speed of sound in air. There are two measuring methods offered in the activity. Depending on an interface a different method can be used
Activity Speed of sound
In this activity students determine the speed of sound in air. There are two measuring methods offered in the activity. Depending on an interface a different method can be used
Activity Speed of sound
 In this activity students explore radioactive decay via a given decay model. Based on the model they determine the function, which describes the radioactive decay. Further they modify the model to describe a decay of a real radioactive isotope e.g. Barium 137m and compare how well the model fits the experimental results.
Activity Radioactive decay
In this activity students explore radioactive decay via a given decay model. Based on the model they determine the function, which describes the radioactive decay. Further they modify the model to describe a decay of a real radioactive isotope e.g. Barium 137m and compare how well the model fits the experimental results.
Activity Radioactive decay